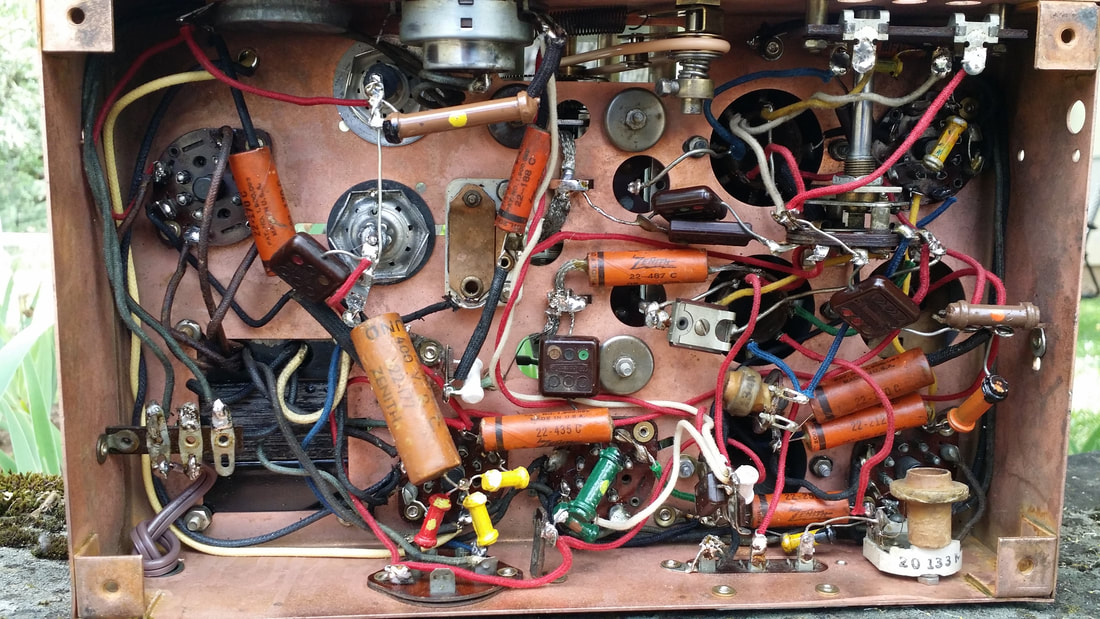
When I purchased this radio off of P-Bay things were Covid-slow. So I decided to do a more in depth restoration of the chassis. Little did I know that Bill Reid was going to donate the rest of his collection - about 3 truck loads, but, more on that later.
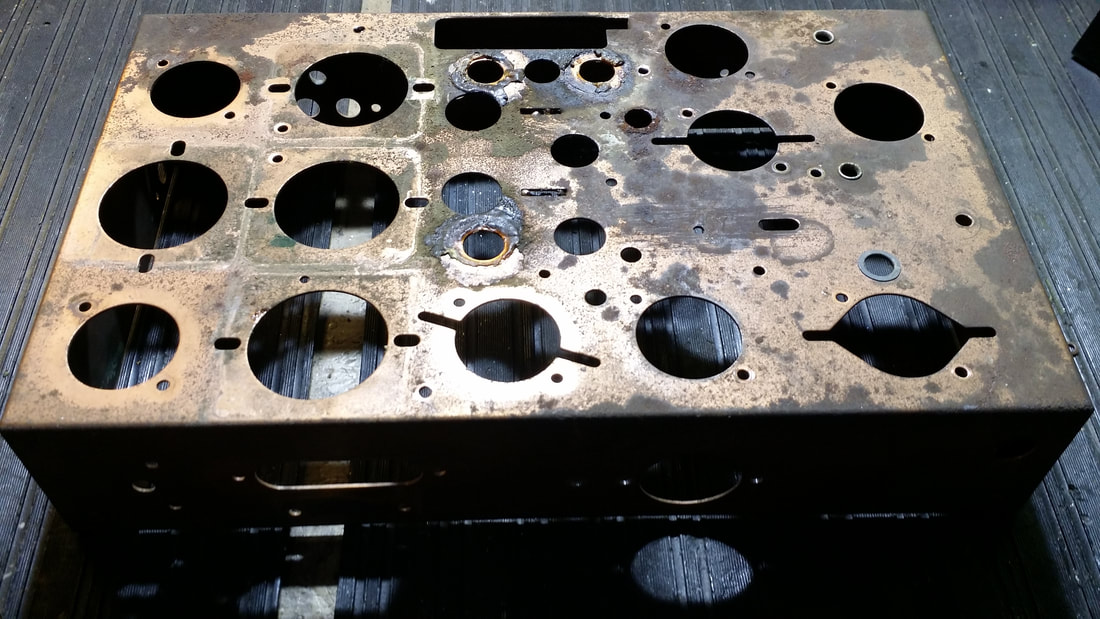
Other than the components, there was also a painted and a plated chassis that often has green, rusty patches like the one above.
I don't like to paint chassis that were originally plated. Besides the issues with ground points being corroded and subsequently , painted, I just don't want a radio that has been altered in that way. SO it was time for MORE FUN WITH CHEMICALS AND ELECTRICITY!
The white powder is washing soda, easily found at WM.
I am avoiding using the term "Anode" here since it can be confusing, ESPECIALLY for a person that is accustomed to working with vacuum tubes. If you want to get it straight in your head, you will have to do some reading and I did not want to bore you here.
The result will be a stream of bubbles coming off of the rusty chassis and a nasty looking film of orange gunk will float to the surface eventually sinking to the bottom.
THE GASSES EMITTED IN THIS PROCESS ARE COMBUSTIBLE and that is why THIS SHOULD ALWAYS BE DONE OUTSIDE. And, if you are not absolutely sure about the technique DON'T DO IT AT ALL.
You might want to fill some of the pitted places with solder - easier said than done. Solder works best on the nickel plate rather than the bare steel. Use an iron - NOT a torch.
I really don't do this all that much (and for that reason, take my advice with a grain of salt - like you usually do). I was happy with the results. Kinda' PINK, but it will be the color of an old penny after a while.
Some people use bolts or screws. I use rivets like the originals, besides, isn't hitting a chassis with a hammer something you have often wanted to do?
BUT WAIT - THERE'S MORE!
Almost every metal component on these chassis are plated with something. The tuning cap is plated with nickel (not the aluminum plates) and the IF transformer cans are plated with zinc. Talk about dissimilar metals! I think that the cans needed to be plated to avoid corrosion of the base metal which seems to be aluminum.
After dealing with the nickel plated components I moved on to a new challenge - zinc plating - which is supposed to be easy(er).
As found
Sanded/cleaned up
Replated
Keep in mind that I am showing you what I did, not telling you to do it.
Below is the configuration I used for plating zinc. The zinc foil, which is very soft/bendable, was purchased for very few $ off of Amazon. The plastic bowl I borrowed from Sue. I don't think that she will miss it - - or want it back. You must use a variable voltage power supply. So the big sucker pictured above will not do.
The IF/RF transformer cans came out a dull grey, just like I expected them to be. During the plating I noticed that the zinc could appear more like a galvanized finish if I deposited less zinc on the base metal. This included the wavy lines and markings typical of a galvanized surface. So if you like that, stop sooner.
Some more notes:
I needed a dielectric to transport zinc ions and water alone will not do - maybe salty water, but I did not try that.
I saw somewhere on the internet (source of all wisdom) that a person could use the washing soda used in stripping the steel to make a solution. I tried this. One way to see if your setup is working is to monitor the amperage on your power supply and watch for the small bubbles. There were bubbles - and bubbles - and nothing else.
So I decided to ditch the washing soda water for clean in which I deposited about 2 tablespoons (use plastic spoon) of battery acid (sulfuric acid) - - do I really need to tell you not to do this? Anyway, now there was some action. At first I needed to set my supply at about 15 volts . BUT, by the time I finished 4 of the cans I had dropped the plating voltage to about 1.5V. (that is one and a half volts)
I suspect that the process formed zinc sulfate in the dielectric which encouraged the transfer of zinc to the plated surface. Had I maintained the 15 volt initial setting, I would have destroyed the can and the foils and - you know - sparks, fire, explosion. So if a person was to try this - and I know that you won't - careful monitoring is absolutely necessary.
Zinc sulfate is a component of fertilizer, but I would not pour this stuff on the flowers. I dispose of chemicals responsibly.
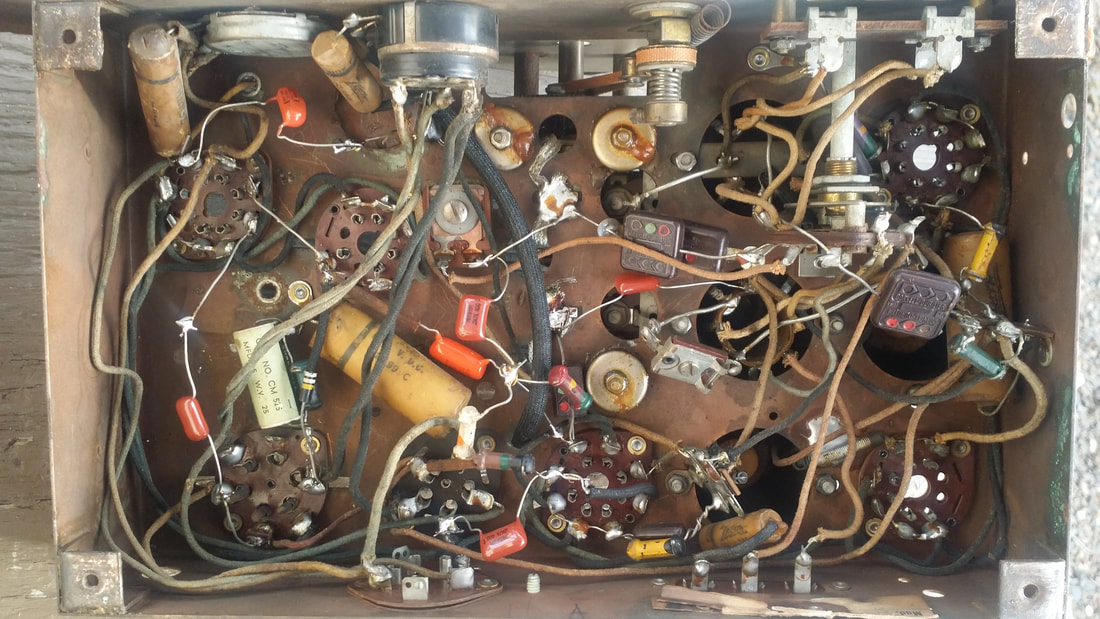
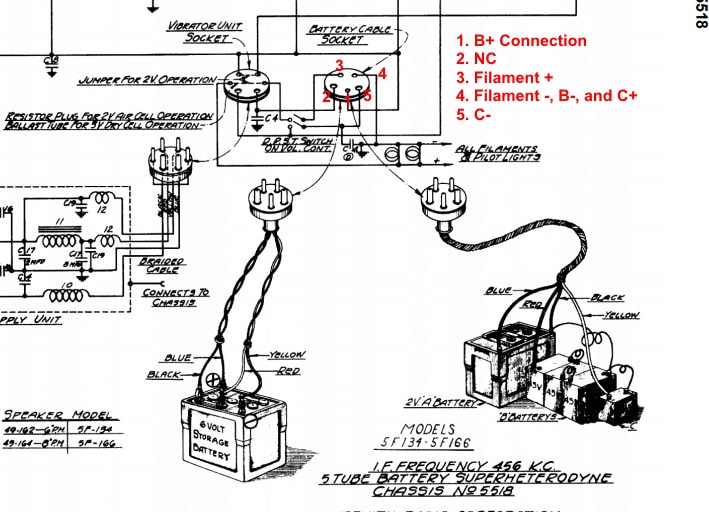
I had always thought that a radio with the brackets and without the vibrator supply was simply missing that supply. But, no, there is another configuration for storage batteries, a setup that would be familiar to users of mid 1920s battery radios.
So it is perfectly normal to have a radio that does not use the top mounted vibrator supply.
Russ
- -and thanks Bill for filling up my shop ;-)
Bill Hennessey has compiled a spreadsheet on the differences between the early model AC chassis and the later one:
In the interest of working out the differences between Chassis 5516 Var. A and Var. B (the original subject of this post) I have created a Google spreadsheet with the stated values for the two known Schematics involved. I've also put in place the values as recorded by bastardbus for the Chassis 5516 Var. B.
There are three tabs in the spreadsheet. One tab for the 5-S-127 Chassis 5516 Var. A (Rider 7-7 Schematic), the second tab for 5-S-127 Chassis 5516 Var. B (no known schematic, values from bastardbus), and the third tab for the 5-A-127 Chassis 5517A (Rider 10-15 Schematic).
Those values listed in Chassis 5516 Var. B and 5517A that substantially differ from Chassis 5516 Var. A are in Red.
https://docs.google.com/spreadsheets/d/1U_oBMYzxQR48MkZR6t7u7yo2bScMciomPMwI2XKKk8E/edit?usp=sharing
I offer this in the hope it may help us to more easily track these capacitor and resistor values and provide some additional guidance to those hoping to restore their Chassis 5517 Var. B sets.
Let me know if there are any additional values or data that should be considered for inclusion here. Right now, I've set this Google spreadsheet to allow comments, feel free to add comments on values that you have found in your own sets.
Thanks!
_________________
Michael Hennessey
mbhdesign






















 RSS Feed
RSS Feed